
 |
What is Benzene?
Benzene was discovered in 1825 by the English scientist Michael Faraday, and in 1842 it was made available in large quantities after it was found that coal tar contains benzene. Large quantities of benzene are now obtained from petroleum. The structure of the benzene molecule is important to organic chemistry. The first to formulate the resonating ring structure described above was the German chemist Friedrich August Kekulé von Stradonitz, in 1865. Benzene's StructureBenzene's structure took 40 years to be solved as no proposed structure could understand how you could get 6 carbon atoms and 6 hydrogens in the same molecule. Back in the 1800s it was understood that carbon had single bonds. Kekulé was the first to be recognised for discovering the structure of benzene. However if you read on this site about Joseph Loschmidt There is a touch of controversy!The cyclic nature of benzene was confirmed by the crystallographer Kathleen Lonsdale. If you have any knowledge of chemistry you will understand that single and double bonds have different lengths. This is because of how bonding works. In Benzene to get a cyclic structure you would need to get 3 double bonds and 3 single bonds. However, how does that work when the bonds are of different lengths? Resonance and DelocalisationAs you might have seen on the aromaticity page, benzene does not behave like an alkene, even though it has double bonds. The answer is actually quite simple yet complex. The usual image you see to represent benzene (a hexagon with a ring in the middle see above), is actually a "resonance hybrid". Below you will see an explanation for this.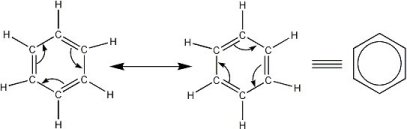
As you can see benzene is actually a hybrid between the two Kekule forms you see above. The explanation lies with the electronic nature of bonds. Single bonds are formed when electrons in line between the atoms interact. These bonds are also known as "s" (sigma) bonds. Double bonds are a mix of a sigma bond and a "p" (pi) bond. The electrons in these bonds actually interact above and below the planes of a sigma bond. In this case, the interactions will be above and below the ring of benzene. The following diagram explains this a little better! 
In the picture above you can see two hypothetical atoms bound together. The dark grey "cloud" is where the sigma electrons lie. As you can see they are directly opposite each other and interact in a straight line along the bond (the black line). The other white and black orbitals sit at a perpendicular angle to the sigma "clouds". You can see that these "clouds" known as orbitals actually sit above and below the atoms. This means that electrons can move freely between the two orbitals. In the next picture I will represent a benzene ring with just p orbitals and attempt to show you what happens. 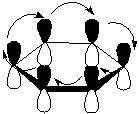
As you can see, because the orbitals are now out of the way of the ring, they can interact above and below the plane in a ring fashion! (See where the ring in the symbol above comes from?). Instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Here is the solution to our problem of not having enough single bonds or not enough double bonds. The lengths of all the bonds in benzene are actually 1.5 times a normal single bond's length. So what happens is that the extra electrons actually strengthen all the bonds in the ring equally! |
 |