
 |
AromaticityWhat is Aromaticity?For those of you who have studied chemistry at school, you might remember that you talked about alkanes and alkenes. Having talked about alkenes, you might remember then moving onto a chemical known as benzene. Benzene does not react like a normal alkene even though it has under the Kekkule form, three sets of double bonds. Unlike an alkene which will give you a dibromoalkane, benzene will only react with a catalyst to give you monosubstituted product (see below). 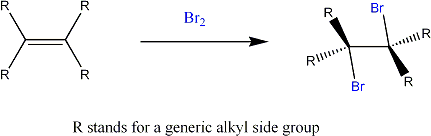
The above reaction shows how an alkene reacts with bromine to give you a dibromoalkane. 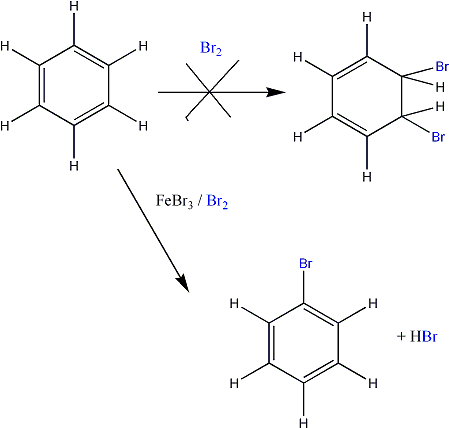
The above diagram shows that you only get a MONOsubstituted benzene. The byproduct is HBr. Remember, a catalyst is not used up in a reaction! So clearly, we have a problem that needs solving. What on earth is happening with this benzene? It looks like an alkene and it has double bonds. Why is it not reacting in the same way? To find out more about benzene click here |
 |