
 |
Henry Edward Armstrong
From 1867 to 1870, Armstrong studied in Germany, at the University of Leipzig. There he studied under Hermann Kolbe who introduced him to aromatic chemistry. He was awarded a Ph.D. for his thesis entitled "Contributions to the history of the acids of the sulphur series, I. On the action of sulphuric anhydride on several chlorine and sulphur compounds." In 1874 he published a book, "Introduction to Organic Chemistry", which is still available in certain libraries for viewing. In 1876, at the age of 28, he was elected as a fellow to the Royal Society and served the Chemical Society in London as secretary, vice-president and president (1893-1895). Armstrong was not content with the work of Kekkule believing that one carbon in the benzene ring directly affected the other five through a central bonding system. As you will see further on; Armstrong actually made an important discovery with his naphthalene structure. Armstrong acknowledged Kekulé’s structure as "being superior to all other symbolic expressions in almost every respect." However he did not agree with it because apparently two meta and two ortho derivatives existed and that it represented benzene as having three pairs of carbon-atoms in "the condition of ethylene". So Armstrong came up with the centric formula. 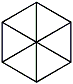 Later Revised To Later Revised To
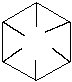 The importance of the bonds not touching in the middle is very significant. Armstrong believed that there were forces acting towards the centre of the molecule. These "affinities" played a part in the ring. With hindsight, what he was talking about was the delocalisation and resonance effect. His formula was almost right but it was not till he expanded his theory to naphthalene (see below) that he made a truly important point. 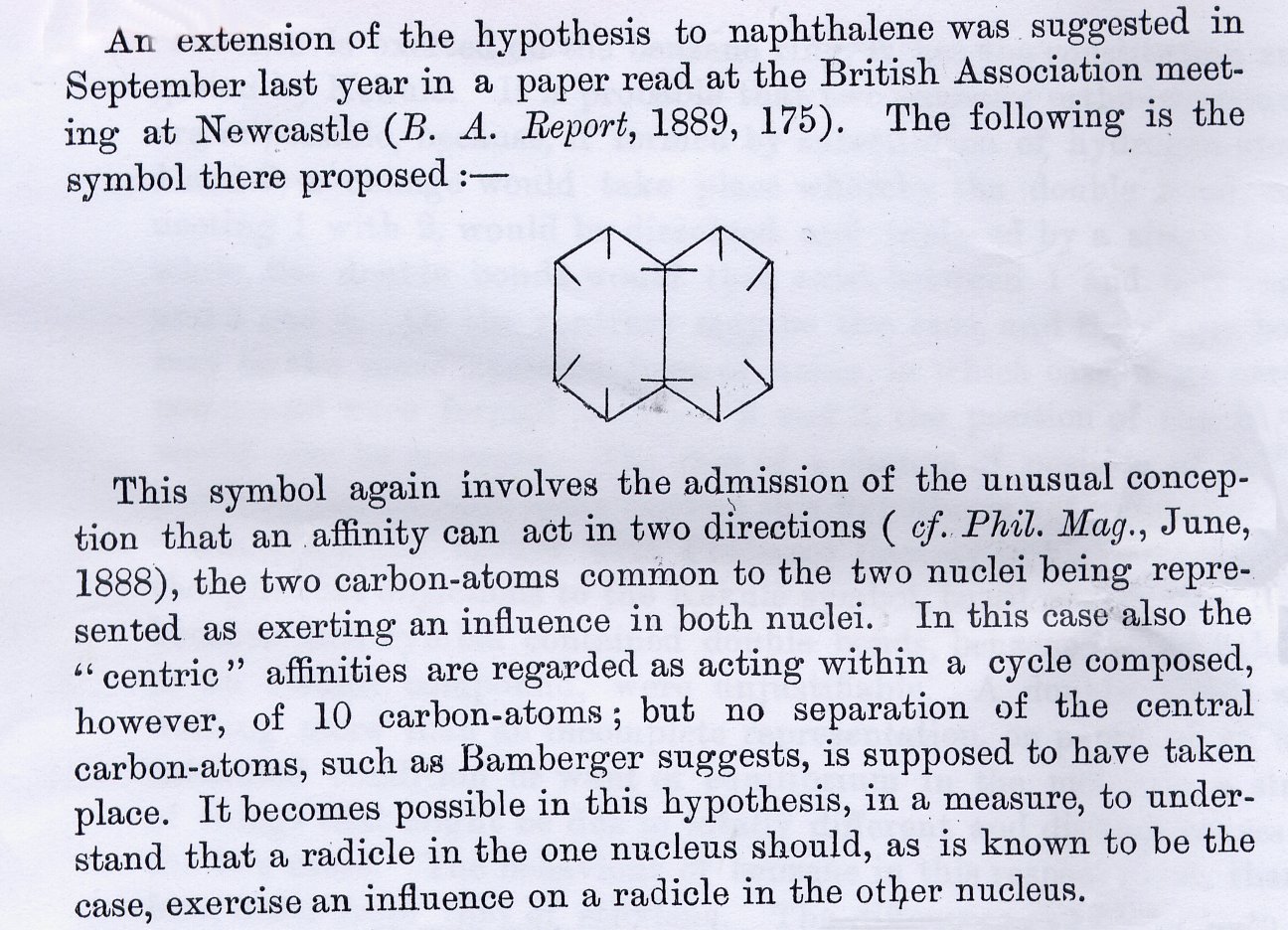
The fact that the two bonds in the middle of the molecule cross is of a very big importance. This shows that Armstrong is not sure of how the centric force works but he does know that his "affinities" have something to do with the molecule’s reactivity. Let us look a little close at the last line in the excerpt. "It becomes possible in this hypothesis, in a measure, to understand that a radicle in the one nucleus should, as is known to be the case, exercise an influence on a radicle in the other nucleus." What this is essentially saying is that for some reason, the "radicle" attached to one of the nuclei (carbon atom in benzene) must affect the other "radical" on the other carbon atom. Now let us use modern day terms and re-write that quote and you will get: "It becomes possible in this hypothesis, in a measure, to understand that a side group (R group) on a carbon, should, as is known to be the case, exercise an influence on the R group on the other carbon atom." Tie that up with "An affinity can act in two directions" and you get the first signs of some quantum mechanics! Of course this is a theory with a few assumptions made. But could it be that Professor Armstrong just did not see that electrons follow a wave pattern? Up to you to decide!! |
 |